eDNA and GIS for Powerful and Scalable Biodiversity Monitoring – Part 1
Did you know that a few drops of water from the ocean can contain traces of hundreds of different organisms? And that these traces can be used to identify the species living in an ecosystem from just a water sample? That is the power of environmental DNA (eDNA), a tool used to characterize and assess biodiversity across ecosystems.
This blog post was written by Beverly McClenaghan (eDNAtec) and Dr. Mohamed Ahmed (Esri Canada). Beverly is the Ecology Lead at eDNAtec, where she oversees field sampling operations as well as analysis and interpretation of eDNA data for projects around the world.
Monitoring biodiversity is increasingly important given the significant and ongoing decrease in global biodiversity, primarily due to human activities. According to WWF’s Living Planet Report 2022, there has been an average 69% drop in monitored mammal, bird, fish, reptile, and amphibian populations since 1970. This loss was primarily due to habitat destruction caused by unsustainable agriculture or logging. There are an estimated 1 million species threatened with extinction, unless action is taken to address the main drivers of biodiversity loss. Such a loss drastically disrupts how ecosystems function, including nutrient cycling, flood control, food provisioning, and more. To combat this, we urgently need scalable tools to survey and monitor the breadth of biodiversity across habitats so that we can better understand the impacts of our activities and take necessary steps to stop and reverse this decline.
Environmental DNA, or eDNA, is DNA left behind by organisms in their environment. Imagine a fish swimming through the ocean: as it moves, it sheds tiny amounts of DNA into the water. By collecting a water sample from an area it swam through, we can capture this DNA, which includes genetic traces from the fish. The sample will also include genetic traces of all other organisms present. Each DNA fragment has a unique sequence that allows us to identify the species it originated from. And it’s not just limited to water – eDNA can also be found in sediment, soil, and even air!

eDNAtec staff collecting water eDNA samples from the Atlantic Ocean. Credit: eDNAtec
eDNA is increasingly being used for biodiversity monitoring across various applications. Due to its high sensitivity, species can be detected from just a few fragments of DNA in a sample, making it a powerful tool for identifying rare organisms, such as endangered species, or for the early detection of harmful organisms, including pathogens and invasive species. Samples can be analyzed to detect all species in an ecosystem — from microbes to mammals and everything in between —making eDNA an efficient and scalable method to monitor whole-ecosystem biodiversity. This is ideal for creating biodiversity baseline inventories and monitoring human impacts, including the effects of infrastructure development and resource extraction activities, as well as ecosystem restoration and the establishment of protected areas. Having information on all taxonomic groups and trophic levels present can empower managers to make informed decisions based on whole ecosystems rather than focusing on a few keystone species. Additionally, eDNA is non-invasive, meaning organisms don’t need to be captured or removed from their environment, ensuring minimal disruption while providing valuable insights into biodiversity. Samples are easy to collect and don’t require experts in the field; with training and the right supplies, anyone can gather eDNA samples.

eDNAtec's high-throughput DNA sequencer, the NovaSeq, being loaded to sequence eDNA samples. Credit: eDNAtec
There are several approaches for collecting and analyzing eDNA samples, tailored to the specific goals and applications of a project. The first step is sample collection, which can involve water, soil, sediment, or air samples, depending on the environment and organisms under study. Samples are generally relatively small volumes, from a few grams of soil to a few litres of water. Once collected, DNA is isolated from the samples and then certain regions of DNA, known as barcode regions, are targeted for analysis. Barcode regions contain DNA sequences that are unique to each species, allowing for precise species identification.
Barcode regions can be analyzed using a few different methods. One approach is quantitative PCR (qPCR), which detects DNA from only one target species using an assay designed for that individual species. This method is ideal when the focus is on one or a few species, as a signal will only be detected if the target species' DNA is present in the sample. Another approach is metabarcoding, which is used when the goal is to study many species or whole biological communities. In metabarcoding, the barcode regions of DNA from broad taxonomic groups, such as animals or eukaryotes, are all sequenced simultaneously. The unique sequences are then used to identify multiple species within the ecosystem. This method is an efficient one for characterizing the diversity of organisms in an ecosystem.
Species identification using DNA sequences relies on reference sequences from known specimens; however, reference sequences are not always available for all species in every ecosystem. Several regional and international efforts are ongoing to fill in the gaps in reference sequence databases, which will increase the capability of eDNA to identify species, particularly in highly diverse and remote ecosystems that are less well studied. In cases where species identifications are not possible due to a lack of reference sequences, unique DNA sequences can still be used to track biodiversity trends over time and space in a taxonomy-free approach.
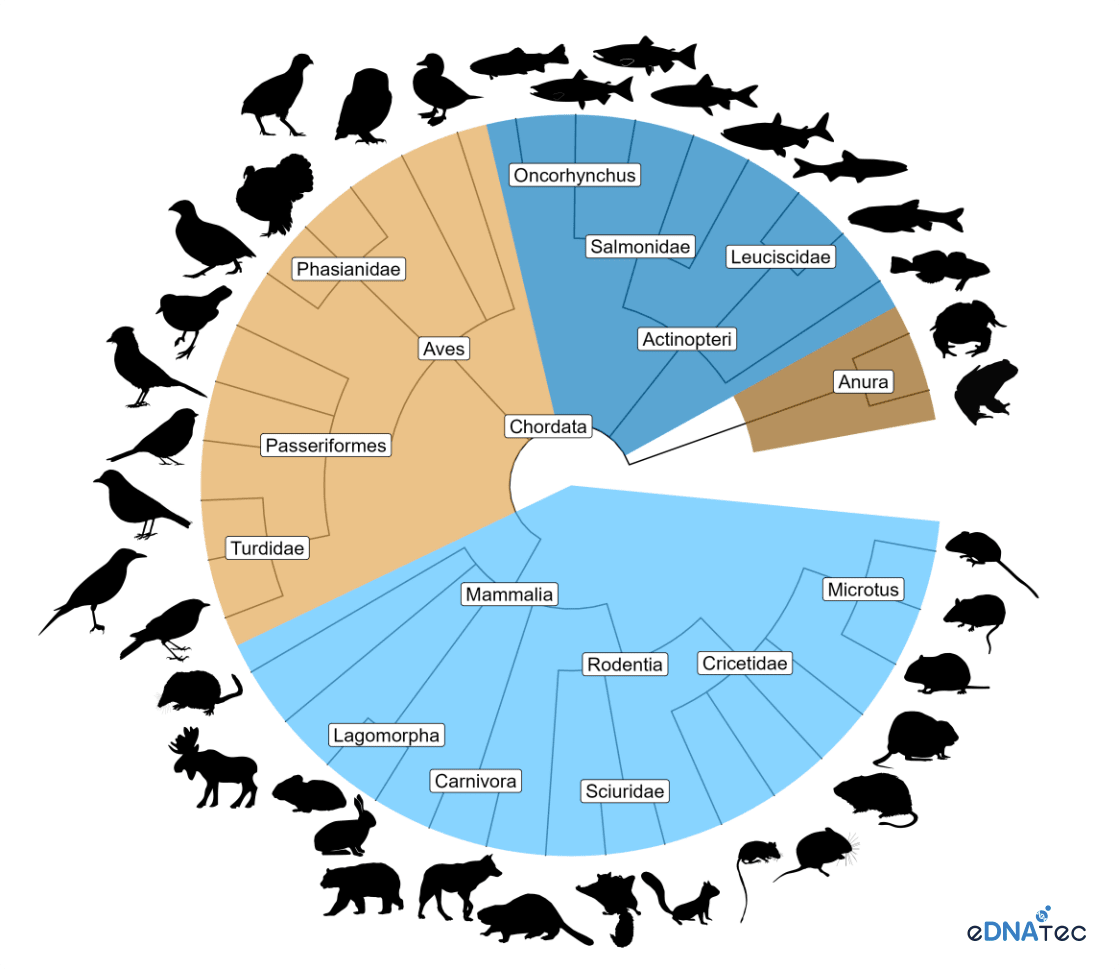
Vertebrate species detected from river water samples collected in British Columbia and analyzed at eDNAtec. The chart demonstrates the diversity of species that can be detected from eDNA samples. The samples also detected many invertebrate species which are not shown here. Credit: eDNAtec
Using the power of high-throughput, next-generation DNA sequencing, analysis of eDNA samples generates a large volume of data yielding insights about species distribution, ecosystem health, and community level changes over time and space. To maximize the impact of these data, they need to be integrated with environmental and geospatial data to appropriately interpret the results and make meaningful conclusions and recommendations.
GIS tools not only enable the precise mapping and visualization of eDNA data, but they also allow researchers to explore how species distributions and ecosystem health vary across different geographic areas. By integrating eDNA data with other environmental and spatial datasets — such as land use, land cover, climate, and topography — we can uncover patterns and correlations that may otherwise be difficult to detect. This integration fosters a more comprehensive understanding of ecosystems, revealing how species interact with their environment and how these interactions evolve over time. The applications of machine learning, artificial intelligence, and other advanced statistical tools further enhance our ability to interpret increasingly complex eDNA biodiversity data, offering new insights into ecological changes. GIS also allows us to model future scenarios, predicting how ecosystems might respond to climate change or habitat destruction, enabling more targeted and effective interventions based on spatially aware eDNA data.
Join us for part 2 to see how GIS tools and eDNA work together to enhance our understanding of aquatic ecosystems!